The science behind calcium carbonate and ocean acidification
The Whirlpool of Ocean Acidification Explained
As Carter (that’s me, your friendly neighborhood science blogger), I spend my days soaking up information like a sponge. More often than not, this knowledge sharing takes place between my daughter Elora and myself, typically during bedtime tales of the vast enigma – the universe. One constant in these narratives, however, is our beloved planet Earth, and some rather worrying developments in our oceans particularly in relation to ocean acidification and the pivotal role calcium carbonate plays. Now, don’t fret. This isn’t a primal scream into the void. It's an in-depth look with a dash of humor and hope sprinkled in – the secret ingredient to my storytelling recipe. Picture it: a simple everyday father imparting nuggets of wisdom to an eight-year-old with an Armstrong-sized dream, all while my Maine Coon cat Bella dozes on my lap, lulled by the soothing tones of geochemistry.
Unraveling The Carbonate Chemistry Module
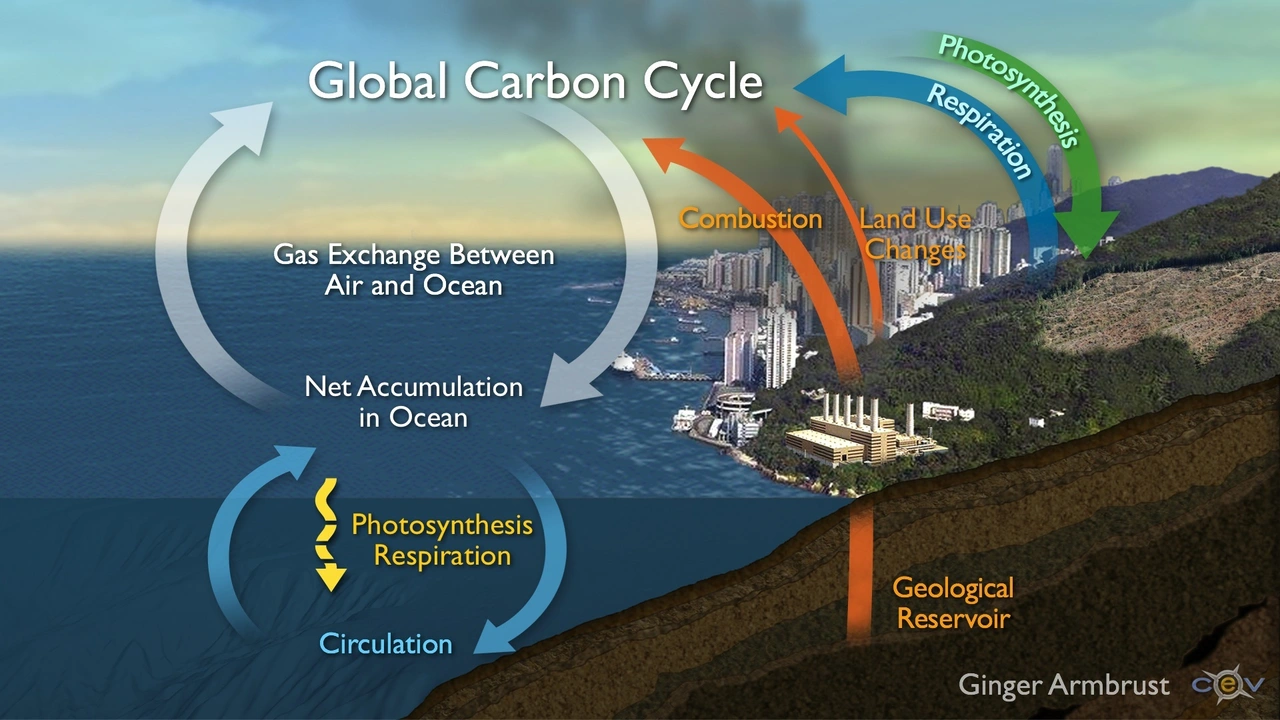
Understanding calcium carbonate and ocean acidification is a bit like understanding why Bella, my cat, insists on sitting on the exact piece of paper that I am looking at. It’s perplexing, slightly comical, but most importantly, there’s a very fascinating science behind it. The calcium carbonate (CaCO3) saga begins with a simple dip in the ocean; an area that covers about 70% of our planet’s surface. Quite like Bella prowling her dominion, our oceans keep the world running in more ways than one. Starting from absorbing about a third of human-made carbon dioxide (CO2) emissions to hosting a myriad of life forms – there’s nary a dull moment under the sea. But adding too much CO2 into the seas? Well, that's where the acidification part comes in, and twist ensues.
Unmasking Ocean Acidification
Consider this: you neatly stack your daughter’s favorite walnut pancakes, drizzle some honey, and suavely slide in some powdered sugar, only to discover it's actually salt. The shock and disappointment, right? Not only does it ruin the taste but adds a bitter layer of frustration. That’s what happens when we pump extreme amounts of greenhouse gases into our atmosphere. This surplus of CO2 integrates with seawater to form carbonic acid, a resultant increase in hydrogen ions (H+), which subsequently leads to a decrease in pH level - a process we refer to as ocean acidification. It’s like turning the oceans into a gigantic salt shaker for marine life – not ideal. The waters aren't getting a pinch; they’re practically drowning in the stuff against their consent.
The Hidden Actor - Calcium Carbonate
Just to keep things right on the edge of a scientific soap opera, I introduce a crucial player – calcium carbonate. Yes, the very calcium carbonate responsible for creating shells and skeletons for a significant chunk of marine organisms – from cabbage-sized foraminifera to GIANT clams! It swoops in like a superhero, balancing out the ocean's pH by combining with surplus hydrogen ions and forming bicarbonate ions (HCO3-). But here’s the catch: in an acidified ocean, those protective shells and skeletons become more likely to dissolve. It’s like being Ultraman: super strong and ready to save the day, but your suit keeps risking dissolution every time you try to fly.
Mitigating Ocean Acidification with a Pinch of Creativity
"In every job that must be done, there is an element of fun" - a mantra that resonates with me, both as a father trying to instill bedtime dental hygiene routines in Elora and a science blogger trying to unpack huge global issues. Ocean acidification induced by excess CO2 emission is a pressing problem. But hey, we humans have a knack for getting ourselves out of the messes we create, don't we? Implementing smarter, eco-friendlier alternatives for energy consumption, initiating massive afforestation drives, and enhancing oceanic resilience through protecting marine ecosystems, are among a few solutions up our sleeves. Let’s face it: we need our oceans in good working condition. They're not just a home to an extraordinary plethora of biodiversity or earth’s natural carbon sink, they’re the ultimate pantry for Bella’s favorite seafood!
Like my adventures in explaining the universe to Elora, this journey through ocean acidification and calcium carbonate's role has been intricate, funny at times, and underpinned with small, digestible tips and facts. This profound appreciation for our blue planet is something I aim to share in my every piece, sized up just right for a friendly chat over coffee - or a father-daughter storytelling session under the stars. And if a common man like me, with a computer for blogging and a cat named Bella perched on my lap, can understand and share this, so can you.